
(March 25, 2020): As our clients and all members of the health care community are acutely aware, the challenges raised by the Coronavirus / COVID-19 outbreak are critical and widespread. Because of these challenges, the Centers for Medicare and Medicaid Services (CMS) and other principal operating components of the United States Department of Health and Human Services (HHS) are issuing frequent guidance to our community.
Additionally, Congress is currently working on the third and large piece of legislation to address both the health care and economic impact of this outbreak. Over the last two weeks, our attorneys have posted a variety of helpful articles on our Firm's website that are directly responsive to these activities. This article provides an update to two pieces of guidance that the CMS recently issued.
I.COVID-19 Outbreak Relief From Certain Quality Reporting Requirements for Clinicians, Providers, Hospitals and Facilities:
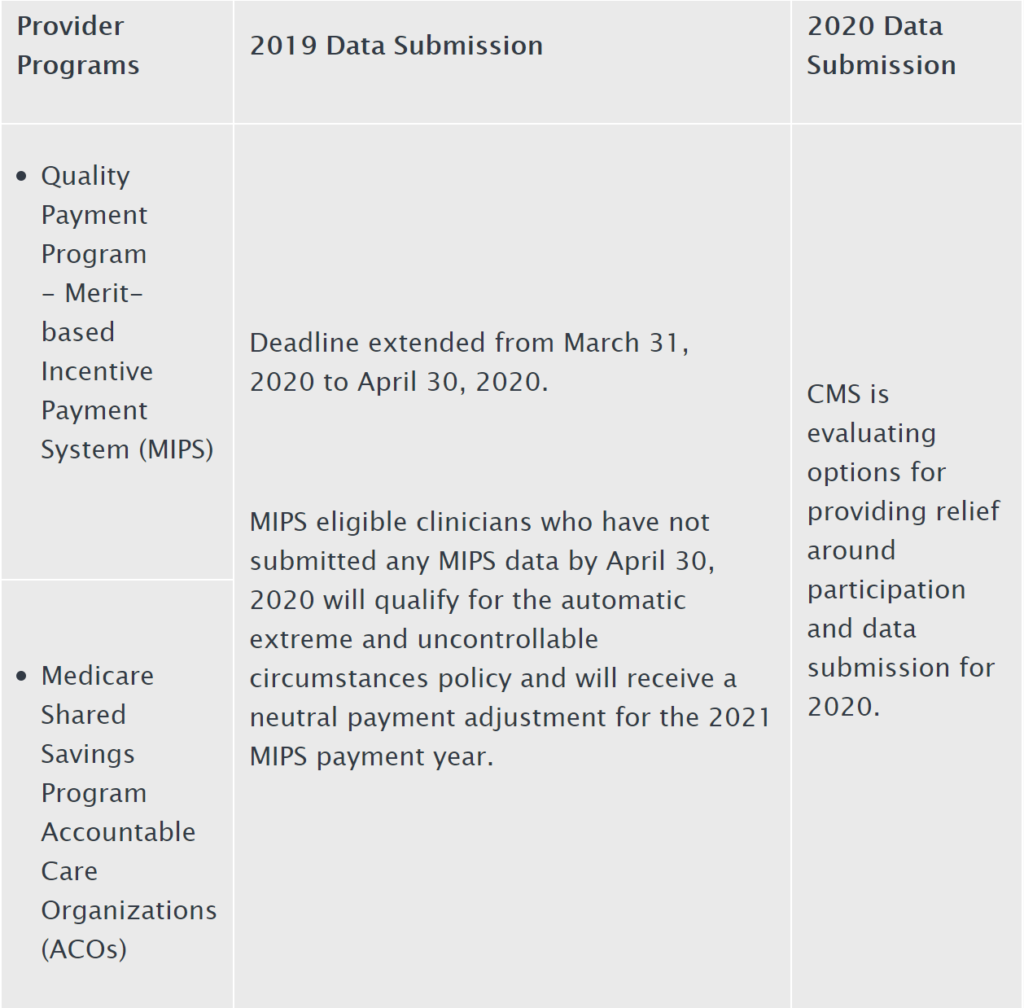
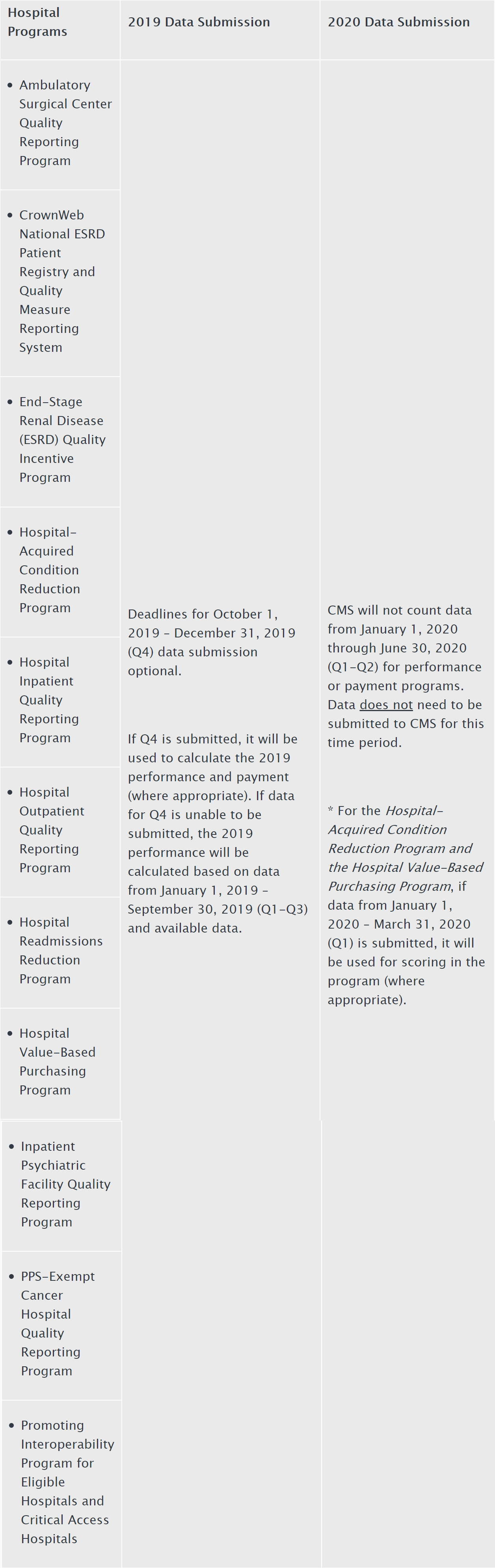
On Sunday, March 22, in response to the COVID-19 outbreak, CMS announced that it was granting exceptions from reporting requirement and extensions for clinicians and providers participating in Medicare quality reporting programs with respect to upcoming measure reporting and data submissions for these programs. The Press Release states that “[s]pecifically CMS is implementing … extreme and uncontrollable circumstances policy exceptions and extensions for upcoming measure reporting and data submission deadlines for the following CMS programs:"

The Press Release further states:
- Submission of data for those programs with submission deadlines in April and May 2020 will be optional based on the facility’s choice to report; and
- CMS will not use data reflecting services provided from January 1, 2020 through June 30, 2020 in calculations for the Medicare quality reporting and value-based purchasing programs.
The Press Release indicates that these actions are being undertaken to reduce data collection and reporting burdens on providers while they are responding to the COVID-19 outbreak pandemic and also because data furnished during this period my not be reflective or certain performance measures during this time, and as a result “seeks to hold organizations harmless for not submitting data during this period.”
The entirety of the Press Release can be found at https://www.cms.gov/newsroom/press-releases/cms-announces-relief-clinicians-providers-hospitals-and-facilities-participating-quality-reporting.
II. New Targeted Plan for Health Facility Inspections:
A. CMS guidance on a short-term re-focusing of the inspection process.
As a result of the recent COVID-19 outbreak, CMS has recognized the increasing stress of the workload being placed on front line clinicians as they care for patients and residents. This is especially true for nursing facilities which face unique and critical challenges in caring for their patients, and where the shortage of supplies of personal protective gear, while true throughout many sectors of the health care system, is especially acute in these facilities. Therefore, on Monday, March 23, CMS released guidance that streamlines the survey process for these and facilities and other providers.
The guidance states that as of the date of publication, according to the Centers for Disease Control and Prevention (CDC), 147 nursing facilities across 27 states have at least one resident diagnosed positive as a result of the COVID-19 outbreak. CMS will be reviewing data as it comes in from the CDC, using that information to identify areas where the virus is likely to strike next and will be targeting inspections accordingly.
B. Overall general framework of the process.
The guidance stresses three action areas:
- CMS will continue responsiveness to immediate jeopardy situations;
- CMS will work with the CDC to identify areas of risk of COVID-19 outbreak spread to ensure that providers are compliant with federal infection control requirements; and
- the guidance contains two tools/protocols which inspectors will use for infection control during this period, and that CMS strongly encourages providers to use as a voluntary self-assessment tool to review their own compliance with federal infection control requirements – one for nursing facilities and one for hospitals and continuing care.
C. Specific actions.
During the next three weeks “only,” federal inspections will be prioritized as follows:
*Complaint inspections. These will be conducted for complaints and facility-reported incidents at the immediate jeopardy level, including allegations such as physical or sexual abuse, neglect, or other conditions that are at the IJ level. Inspectors will use a streamlined Infection Control review tool regardless of the IJ allegation.
*Targeted Infection Control Inspections. Federal and state inspectors will be conducting targeted infection control inspections for providers identified by CMS in collaboration with the CDC using a streamlined review checklist. The guidance states that providers will receive immediate feedback to enable them to address shortcomings.
*Self-Assessment. The guidance includes an infection control checklist to allow for self-assessments in this area.
Additionally, the guidance states that:
*Standard inspections of nursing homes, hospitals, home health agencies, intermediate care facilities for individuals with intellectual disabilities, and hospices and revisit inspections not associated with Immediate Jeopardy will not be conducted.
*CMS will prioritize IJ investigations over recertification surveys for CLIA laboratories.
*Absent IJ, CMS will utilize enforcement discretion.
*Initial inspections will be conducted with current guidance and prioritization.
During this period, CMS has also delayed certain enforcement activities as set forth in the quoted section from the CMS guidance document, below:
“ 4.a. For pending enforcement cycles during the prioritization period where the provider is currently not in substantial compliance or has not had a revisit survey to verify substantial compliance, and a per day civil money penalty (CMP), or DPNA (for nursing homes) or SPNA (for HHAs) was imposed for noncompliance that occurred prior to the prioritization date of surveys: These remedies will be suspended (stopped) as of the start of the survey prioritization date. In other words, the CMP will stop accruing and the DPNA/SPNA will end as of the suspension date. Additionally, CMS will not impose any new remedies to address noncompliance that occurred prior to the start of the survey prioritization period. NOTE: This does not apply to unremoved IJs. Enforcement actions will proceed as usual per the SOM for unremoved IJ deficiencies. CMS will issue guidance on how to reconcile these actions in the next few weeks.
b. For pending enforcement cycles during the prioritization period where the provider is currently not in substantial compliance or has not had a revisit survey to verify substantial compliance, and for pending enforcement cycles with new noncompliance cited after the issuance of this memo, and a per day CMP, or DPNA (for nursing homes) or SPNA (for HHAs) was imposed for IJ level noncompliance (where the IJ has not been removed): Surveyors will follow normal policies and procedures for removing the IJ. CMS will also follow normal policies and procedures for imposing enforcement remedies for remediating the noncompliance. For example, for noncompliance cited at the IJ level, that has not been removed at the time of the survey exit, the CMS Office will impose an enforcement remedy (e.g., CMP, 23 day termination), and the state surveyors will conduct a revisit survey. On the revisit survey, surveyors will either verify substantial compliance, or cite noncompliance at a lower level if warranted.
i. If the IJ noncompliance is reduced and cited at level 3 (LTC) or condition level (non-LTC), an onsite revisit survey will not be conducted during the prioritization period, and these cases will be held. CMS will issue guidance on how to impose enforcement and verify compliance with these in the next few weeks (see 2.c. [elsewhere in the full CMS guidance document]).
ii. If the IJ noncompliance is reduced and cited at level 2 (LTC) or standard level (non-LTC), facilities and survey agencies would verify compliance through normal procedures through a desk review (see 2.d. [elsewhere in the CMS guidance document]). However, CMS should not impose remedies during the prioritization period for any noncompliance that was identified before or after the start of the survey prioritization period, unless the noncompliance is an unremoved IJ.
c. The three-month mandatory DPNA and six-month mandatory termination (nursing homes) for not being in substantial compliance (for nursing homes and HHAs) will not take place, and be deferred for an evaluation at a later date. However, enforcement actions related to IJ remain and continue under normal procedures.
d. If CMS has previously imposed an alternative sanction (e.g., SPNA, CMP) on a HHA for noncompliance identified prior to the suspension, the six-month mandatory termination will not take place, and be deferred for an evaluation at a later date.
e. For existing CLIA enforcement cases where a civil money penalty (CMP) per day of non-compliance was imposed, accrual of CMP will stop as of the survey COVID-19 suspension date. CMS will issue guidance on how to reconcile these actions in the next few weeks. Other CLIA enforcement actions that have been initiated will be handled on a case-by-case basis with consultation DCLIQ managers and staff.
Due to its breadth, we have provided the following links to CMS guidance:
*Fact Sheet: https://www.cms.gov/newsroom/fact-sheets/kirkland-washington- update-and-survey-prioritization-fact-sheet
*Full Guidance Document, including Infection Control Self-Assessment Tools: https://www.cms.gov/files/document/qso-20-20-allpdf.pdf-0
D. Other activity involving state Medicaid programs.
Additionally, on March 24, CMS granted 11 states’ Medicaid requests for 1135 waivers that allow greater flexibility in the administration of their programs. We expect to provide a more detailed discussion of this event shortly; however, in the interim, we strongly suggest that all health care entities participating in the Medicaid program check with the states in which they operate to determine whether those states have received such a waiver, and if so, review the waiver to determine any impact that it might have on them. As we all know, as the full scope of the COVID-19 outbreak develops, both Federal and State regulators will be actively monitoring the adverse impact that this national emergency is having on health care providers and suppliers.
III. Conclusion -- Responding to the COVID-19 Outbreak:
As a final remark, we wish to emphasize our awareness that the COVID-19 outbreak is a highly stressful situation that changes almost minute by minute. We also wish to emphasize that as a society, we are all in this together and that we will get through this. Right now, we need to do everything in our power to protect our residents, patients, and staff (and our loved ones and selves). At Liles Parker, we will do our utmost to help you in this process by keeping you up to date of major developments and are available to respond to your inquiries during this period.

Michael Cook is a Partner and Co-chair for the Health Care Group at Liles Parker PLLC. He has more than 40 years representing virtually every form of health care entity in a wide variety of matters across this country, currently is a member of the Board that oversees Virginia’s Medicaid program by appointment of the Governor, has advised a number of candidates for public office at both the state and national levels on health care issues, and has served on health care transition teams for several Governors. Anyone with questions on the issues discussed in this paper should feel free to contact Michael. He can be reached at either (202) 298-8750 (office) or (202) 361-2508 (cell) or mcook@lilesparker.com.
