(September 25, 2025): Medicare providers and beneficiaries will likely face a number of new challenges over the next five years. As you will recall, the Centers for Medicare and Medicaid Services (CMS) Innovation Center has set a goal of having 100% of Traditional Medicare (also commonly referred to as “Original Medicare”) beneficiaries move over to Medicare Advantage plans by 2030.[1] This transition is being promoted despite the fact that Medicare Advantage plans have repeatedly been found to be more costly, per enrollee, than Traditional Medicare.[2] Depending on your perspective, this may be bad news. Medicare Advantage plans have been repeatedly criticized by providers and beneficiaries alike. One of the main criticisms levied at Medicare Advantage plans has been the fact that burdensome prior authorizations for care are commonly required for more costly services and procedures. Unfortunately, CMS is now examining whether it makes sense to impose prior authorization requirements in the Traditional Medicare / Original Medicare program. Beginning January 1, 2026, healthcare providers in a limited number of states will need to seek prior authorization for more than a dozen costly services and medical procedures. CMS is hoping that the imposition of prior authorization requirements will help curb unnecessary medical services while promoting transparency and accountability. This initiative is known as the “Wasteful and Inappropriate Service Reduction (WISeR) Model.” This article examines the WISeR Model initiative and discusses the impact it is likely to have on affected Medicare participating providers.
I. Overview of the WISeR Model:
When discussing the WISeR Model, CMS characterizes “wasteful medical care spending” as “expenditures that could be reduced or eliminated without negatively impacting the quality of care or health outcomes.” CMS estimates that an estimated 25% of total healthcare spending in the United States is wasteful. The WISeR Model initiative is intended to significantly reduce wasteful spending being paid by the Traditional Medicare / Original Medicare program. Here are the basic tenets of the initiative:
- Starting and Ending Dates of the WISeR Model Initiative. In late June 2005, CMS released plans for its Innovation Center to institute the WISeR Model initiative beginning January 1, 2026.[3] CMS intends to implement the WISeR Model over a 6-year period. This 6-year period will be broken down into two 3-year agreement periods with contractors selected to administer the initiative.[4]
- Geographic Scope. CMS plans to initially limit the WISeR Model initiative to six states. These states include New Jersey, Ohio, Oklahoma, Texas, Arizona, and Washington. It is important to remember that this is merely CMS’s first effort to introduce prior authorization requirements into the Traditional Medicare / Original Medicare program. We fully anticipate that CMS will expand prior authorization mandates to other states at the conclusion of this initiative, if not before.
- Service Categories / Procedures Impacted. The services subject to prior authorization range from electrical nerve stimulators to skin substitute applications. While a final list of affected CPT codes will be issued prior to the start of the program, CMS will release a full list of affected CPT codes before the program's start. A tentative list of the affected service / procedure categories is set out below.
Service Categories / Procedures Impacted Applicable Coverage and Payment Guidance Electrical Nerve Stimulators NCD 160.7[5] Sacral Nerve Stimulation for Urinary Incontinence NCD 230.18[6] Phrenic Nerve Stimulator NCD 160.19[7] Deep Brain Stimulation for Essential Tremor and Parkinson’s Disease NCD 160.24[8] Vagus Nerve Stimulation NCD 160.18[9] Induced Lesions of Nerve Tracts NCD 160.1[10] Epidural Steroid Injections for Pain Management / Excluding Facet Joint Injections L39015, L39242, L36920[11] Percutaneous Vertebral Augmentation (PVA) for Vertebral Compression Fracture (VCF) L34106, L38201, L35130[12] Cervical Fusion L39741, L39762, L39793[13] Arthroscopic Lavage and Arthroscopic Debridement for the Osteoarthritic Knee NCD 150.9[14] Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea L38307, L38312, L38385[15] Incontinence Control Devices NCD 230.10[16] Diagnosis and Treatment of Impotence NCD 230.4[17] Percutaneous Image-Guided Lumbar Decompression for Lumbar Spinal Stenosis NCD 150.13[18] Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds L35041[19] Wound Application of Cellular and / or Tissue Based Products (CTPs), Lower Extremities L36690[20]
II. CMS Contractors (Participants) Responsible for Implementing the WISeR Model Initiative:
Under the WISeR Model, CMS’s Innovation Center plans to pay private contractors to process prior authorization requests related to the targeted service categories set out in the above chart. These private contractors are referred to as “participants.”[21]
- How Will Participants Conduct Reviews of Requests for Prior Authorization? As discussed in the Federal Register, CMS is requiring that participants employ a:
“. . . technology assisted prior authorization process to help ensure that all relevant clinical and medical documentation requirements are met before services are rendered to beneficiaries and before claims are submitted for payment.”[22]
In other words, contractors selected to serve as participants are expected to develop and employ artificial intelligence (AI) tools that have been designed to review a Medicare beneficiary’s clinical records, test results and diagnoses in order to determine whether a request for prior authorization should be approved. The AI tools employed by participants will compare the applicable coverage and payment requirements set out under NCDs and / or LCDs when approving or denying a prior authorization request.
- How Will Participants be Paid? Notably, participants will be paid based on the amount of money that the contractor is able to save the government. As CMS states in the Federal Register:
“The WISeR model will focus on testing the implementation of prior authorization and pre-payment review for specific selected services that will be performed by third party entities leveraging enhanced technologies, that would be paid under a novel payment approach where the model participants are compensated based on a share of averted expenditures.” [23] (Emphasis Added).
Simply stated, under this payment structure, participants will be paid more if they deny more prior authorization requests. Our concern is that this approach improperly incentivizes the contractors to employ AI tools that are overly restrictive, thereby resulting in the denial of medical services and procedures that are, in fact, medically necessary and appropriate.
III. Health Care Providers Review Options Under the WISeR Model Initiative:
While CMS describes the WISeR prior authorization process as “voluntary,” that characterization is somewhat misleading. If a provider chooses not to submit a request for prior authorization and decides to perform the service without obtaining prior authorization, the claim submitted to the MAC for payment will be flagged sent to the model participant for pre-payment review.[24] In other words, the services identified for review in the above chart will be either be reviewed by the participant’s AI tools (through the WISeR Model prior authorization process) OR it will be subjected to clinical review by a member of the model participant’s team to ensure that the services qualify for coverage and payment. Even if a provider submits the claim directly to the MAC, when it is forwarded to the model participant it may also be assessed by the contractor’s AI review tools.[25] In order to conduct this review, a provider will likely receive a request for a copy of all supporting documentation. The following flow chart[26] illustrates the review options available to providers:
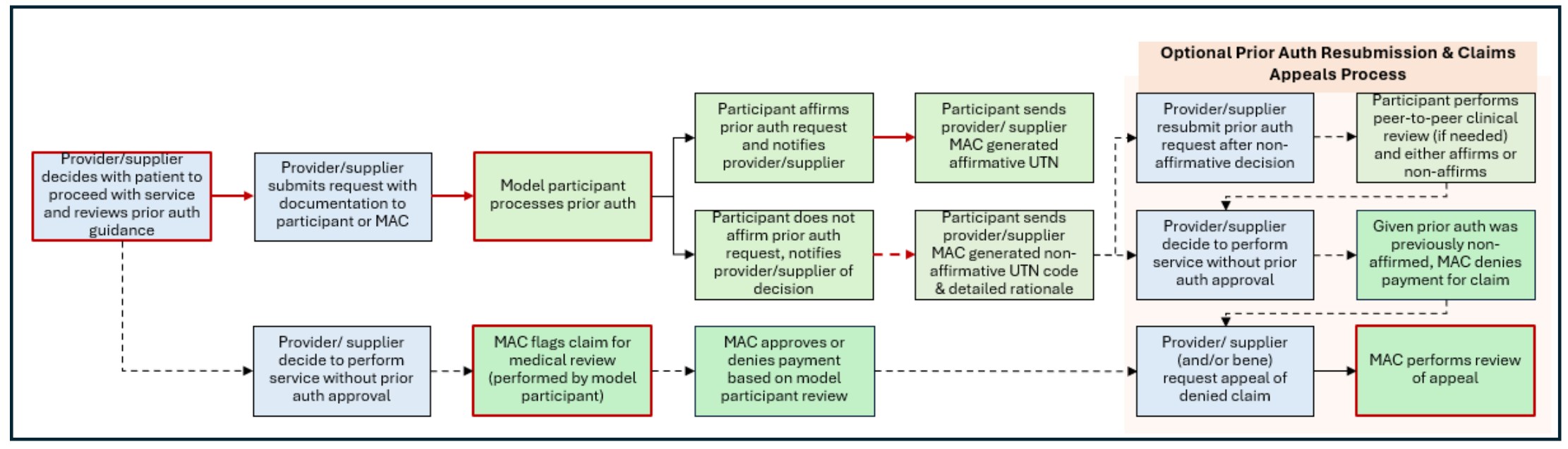
IV. Provider Concerns Regarding the WISeR Model Initiative:
Not surprisingly, a number of concerns have been identified with respect to the WISeR Model initiative. Several of these concerns are discussed below:
- AI Reviews and Assessments are Still a Work in Progress. Prior to being audited, most providers believe that that their documentation is both compliant with applicable regulatory and LCD / NCD requirements. Unfortunately, that isn’t always the case. Typographical errors, the use of casual vernacular, missing words and other mistakes can wreak havoc on an AI’s ability to properly assess medical records and make fully informed recommendations regarding treatment and / or prior authorization. Researchers examining the accuracy of AI applications with respect to clinical decision making recently observed the following:
“These findings indicate that LLMs[27] take nonclinical information into account for clinical decision-making in previously unknown ways. It brings to light the need for more rigorous studies of LLMs before they are deployed for high-stakes applications like making treatment recommendations, the researchers say.” (Emphasis Added).[28]
- Prior Authorization Requirements Result in Delays in Treatment. A 2024 survey by the American Medical Association (AMA) of 1,000 practicing physicians found that 94% reported that prior authorization delays necessary patient care. Moreover, the survey noted that over 80% of the physicians stated that prior authorization requirements often lead to patients deciding to abandon treatment. This burden results in negative impacts on patient outcomes, increased healthcare costs, and significant administrative work for physicians.[29]
- Has the Guidance Used by AI Systems to Assess Requests for Prior Authorizations Been Properly Vetted? While NCDs[30] have gone through a public notice and comment process, that is not necessarily the case with all of the LCDs that have been published by Medicare Administrative Contractors (MACs). A number of LCDs have only recently gone through the public notice and comment process. On February 12, 2019, CMS amended its Medicare Program Integrity Manual (MPIM), §13.2.4, titled "Proposed LCD."[31] As the MPIM provides, in part:
"All proposed LCDs, with limited exceptions noted below, must follow the LCD process outlined in 13.2 of this manual, consisting of consultation, publication of proposed LCD, open meeting concerning the proposed policy, opportunity for public comment in writing, publication of a final LCD that includes a response to public comments received and notice to public of new policy 45 days in advance of the effective date. . .” (Emphasis Added).
To the extent that a model participant will be applying an LCD to the services that are subject to prior authorization, it may be helpful for you to research the history of the LCD at issue. This may assist you when appealing claims denials.
- Claims Denials Resulting from the WISeR Prior Authorization Reviews Can Lead to Adverse Collateral Consequences. Pursuant to 42 CFR § 424.535(a)(21), a pattern or practice of ordering medically unnecessary Part B service can lead to the revocation of a provider’s billing privileges.
IV. Conclusion:
The WISeR Model initiative represents a significant departure from the way Traditional Medicare / Original Medical services have historically been handled. Requiring prior authorization of certain services or procedures is likely to result in a number of unexpected claims denials. Are your services subject to the WISeR Model review process? Give us a call. Our attorneys are experienced in challenging complex Medicare claims denials.

- [1] See CMS’s article titled “The CMS Innovation Center’s Strategy to Support High-quality Primary Care.” (June 9, 2023).
- [2] Medicare Payment Advisory Commission (MedPac) report to Congress titled “The Medicare Advantage program: Status Report.” (March 2024). As the report states on page 372:
“In 2024, we project that MA plan payments (including rebates that finance extra benefits) remain far above what Medicare would have paid for similar beneficiaries in FFS, continuing the trend of higher levels of payment throughout the history of Medicare managed care. We estimate that Medicare spends 22 percent more for MA enrollees than it would spend if those beneficiaries were enrolled in FFS Medicare, a difference that translates into a projected $83 billion in 2024.” (Emphasis Added).
- [3] See CMS’s press release titled “CMS Launches New Model to Target Wasteful, Inappropriate Services in Original Medicare.” (June 27, 2025).
- [4] “Medicare Program; Implementation of Prior Authorization for Select Services for the Wasteful and Inappropriate Services Reduction (WISeR) Model.” 90 Fed. Reg. 28749, 28750 (July 1, 2025).
- [5] National Coverage Determination (NCD) NCD 160.7, “Electrical Nerve Stimulators.”
- [6] NCD 230.18, “Sacral Nerve Stimulation For Urinary Incontinence.”
- [7] NCD 160.19, “Phrenic Nerve Stimulator.”
- [8] NCD 160.24, “Deep Brain Stimulation for Essential Tremor and Parkinson’s Disease.”
- [9] NCD 160.18, “Vagus Nerve Stimulation.”
- [10] NCD 160.1, “Induced Lesions of Nerve Tracts.”
- [11] Local Coverage Determinations (LCDs) L39015, L39242, L36920, “Epidural Steroid Injections for Pain Management / Excluding Facet Joint Injections.”
- [12] LCDs L34106, L38201, L35130, "Percutaneous Vertebral Augmentation (PVA) for Vertebral Compression Fracture (VCF).”
- [13] LCDs L39741, L39762, L39793, “Cervical Fusion.”
- [14] NCD 150.9, “Arthroscopic Lavage and Arthroscopic Debridement for the Osteoarthritic Knee.”
- [15] LCDs L38307, L38312, L38385, “Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea.”
- [16] NCD 230.10, “Incontinence Control Devices.”
- [17] NCD 230.4, “Diagnosis and Treatment of Impotence.”
- [18] NCD 150.13, “Percutaneous Image-Guided Lumbar Decompression for Lumbar Spinal Stenosis.”
- [19] LCD L35041, “Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds.”
- [20] LCD L36690, “Wound Application of Cellular and / or Tissue Based Products (CTPs), Lower Extremities.”
- [21] In late June 2025, CMS issued a “Request for Applications” which sets out the duties and responsibilities of contractors (referred to as “participants” in the document) interested in being selected to administer the WISeR Model initiative.
- [22] 90 Fed. Reg. 28749, 28751 (July 1, 2025).
- [23] 90 Fed. Reg. 28749, 28750-28751 (July 1, 2025).
- [24] 90 Fed. Reg. 28749, 28752 (July 1, 2025).
- [25] Ibid.
- [26] CMS “Request for Applications,” Page 16.
- [27] A "large language model" (LLM) is an advanced AI system trained on a massive amount of text data to understand, summarize, translate, and generate human-like language. They are a form of deep learning, a subset of AI that uses algorithms to recognize complex patterns in large datasets.
- [28] MIT News. See their June 23, 2025 article titled “LLMs factor in unrelated information when recommending medical treatments,” citing a research paper titled “The Medium is the Message: How Non-Clinical Information Shapes Clinical Decisions in LLMs,” presented at the 2025 ACM Conference on Fairness, Accountability, and Transparency.
- [29] See “2024 AMA Prior Authorization Physician Survey.”
- [30] CMS is responsible for issuing NCDs. provides multiple opportunities for public feedback and participation.
- [31] Medicare Program Integrity Manual (MPIM), §13.2.4, titled "Proposed LCD.”
