The Drug Enforcement Administration (DEA) was first established in 1973[1] and is an investigative / enforcement component of the U.S. Department of Justice. The DEA is the primary federal agency that has been tasked with the enforcement of laws governing the illegal sale, distribution, manufacturing or use of drugs. The primary statute enforced by the DEA is the Controlled Substances Act[2] (CSA). The CSA sets out Federal drug policy with respect to both legal (pharmaceutical) and illegal controlled substances[3] and essentially serves as the legal cornerstone of our nation’s ongoing efforts to regulate the safe manufacture, distribution, importation, possession and use of drugs and controlled substances. Furthermore, the DEA is responsible for ensuring that controlled substances are strictly monitored and regulated so that any transactions involving controlled substances take placed with the “Closed System” of distribution. Under this closed system:
“. . . all legitimate handlers of controlled substances – manufacturers, distributors, physicians, pharmacies, and researchers – must be registered with DEA and maintain strict accounting for all distributions.”[4]
I. The Interplay Between Federal and State Controlled Substances Laws:
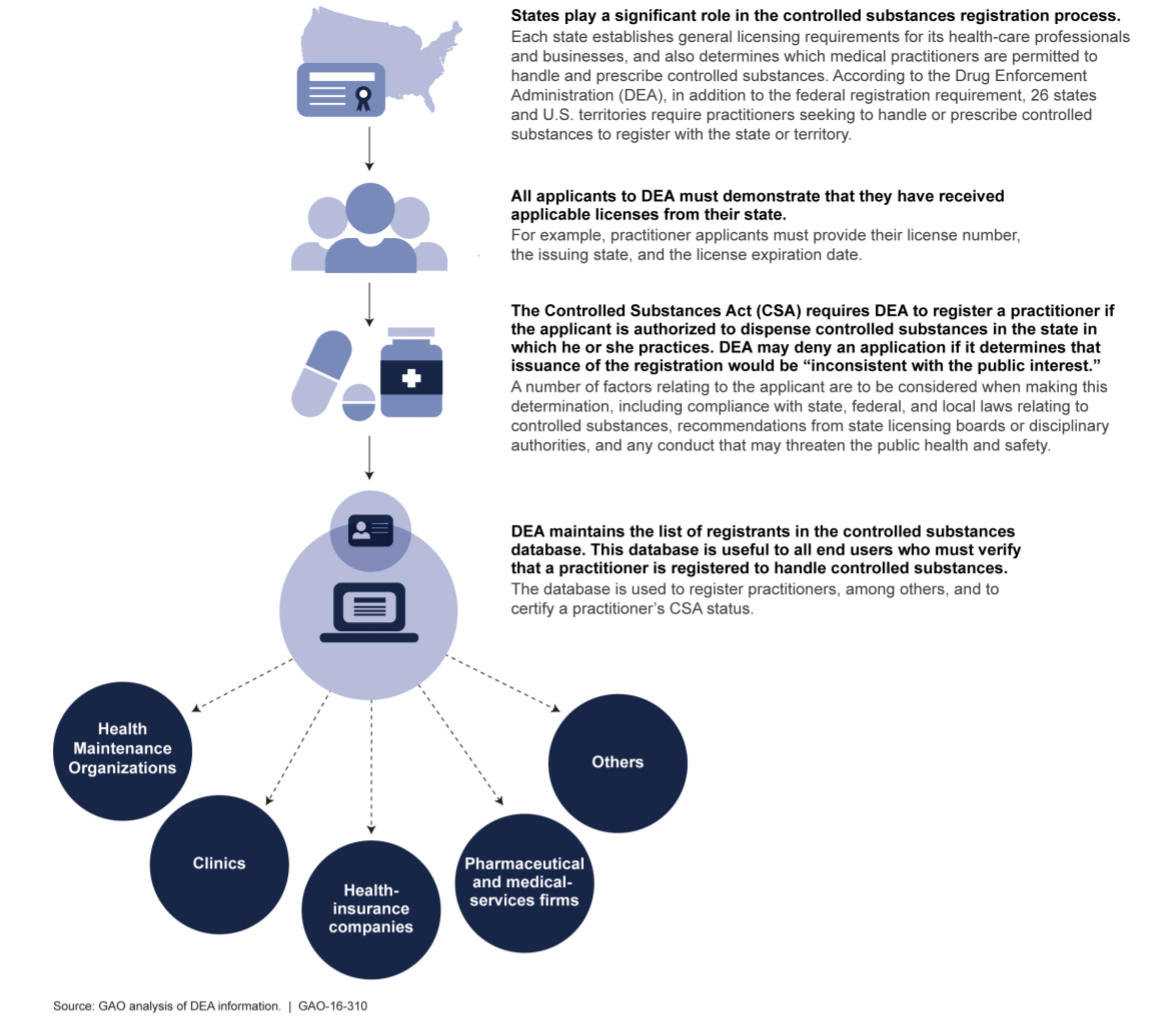
Federal controlled substances laws are intended to work in conjunction with State controlled substance statutes. DEA agents, investigators and enforcement staff work closely with their State, county and local counterparts to safeguard the public and to make sure that pharmaceutical controlled substances are only prescribed, administered, dispensed and used for legitimate medical purposes in accordance with applicable federal and state laws. DEA representatives also work closely with state professional licensing boards responsible for regulating the actions of physicians, nurse practitioners, physician assistants, dentists, oral surgeons, veterinarians and other licensed medical professionals with prescribing authority.
II. DEA Certificate of Registration Basics
Federal law requires that qualified “practitioners”[5] must obtain a DEA Certificate of Registration in order to handle controlled substances. Applicable State laws also govern the scope of controlled substance related activities in which a DEA registered practitioner may engage. Therefore, a practitioner is required to adhere to both Federal and State controlled substance statutes and regulations. To the extent that applicable Federal and State laws are inconsistent or may conflict, a practitioner is required to comply with whichever requirement is more stringent or restrictive.
IV. What Does it Mean if the Issuance of a DEA Certificate of Registration Would be “Inconsistent with the Public Interest”?
There are a number of statutory reasons that may serve as a basis for denying an applicant’s DEA Registration. Of these reasons, the argument that the issuance of a DEA Registration would be “inconsistent with the public interest” is most often cited by the government when a denial has been proposed. What does this mean? Under 21 U.S.C.§ 823(b), the following factors are to be considered when making a determination of whether the issuance of a DEA Registration would be inconsistent with the public interest:
(1) maintenance of effective control against diversion of particular controlled substances into other than legitimate medical, scientific, and industrial channels;
(2) compliance with applicable State and local law;
(3) prior conviction record of applicant under Federal or State laws relating to the manufacture, distribution, or dispensing of such substances;
(4) past experience in the distribution of controlled substances; and
(5) such other factors as may be relevant to and consistent with the public health and safety.
V. What Happens if Your Application for a DEA Certificate of Registration is on Track to be Denied?
After you complete and submit your application for a DEA Certificate of Registration, the agency will conduct a review of your application (along with any other relevant information that it has identified) in order to make a determination of whether your application should be approved. If the Administrator is unable to approve of your application, the Administrator is required to issue an “Order to Show Cause” why your application for registration should not be denied, revoked or suspended.[6] An Order to Show Cause is required to:
- (2) An order to show cause under paragraph (1) shall—
(A) contain a statement of the basis for the denial, revocation, or suspension, including specific citations to any laws or regulations alleged to be violated by the applicant or registrant;
(B) direct the applicant or registrant to appear before the Attorney General at a time and place stated in the order, but not less than 30 days after the date of receipt of the order; and(C) notify the applicant or registrant of the opportunity to submit a corrective action plan on or before the date of appearance.
- (3) Upon review of any corrective action plan submitted by an applicant or registrant pursuant to paragraph (2), the Attorney General shall determine whether denial, revocation, or suspension proceedings should be discontinued, or deferred for the purposes of modification, amendment, or clarification to such plan.
- (4) Proceedings to deny, revoke, or suspend shall be conducted pursuant to this section in accordance with subchapter II of chapter 5 of title 5. Such proceedings shall be independent of, and not in lieu of, criminal prosecutions or other proceedings under this subchapter or any other law of the United States.
- (5) The requirements of this subsection shall not apply to the issuance of an immediate suspension order under subsection (d).
VI. How Should You Respond to an Order to Show Cause?
After being served with an Order to Show Cause, you will have 30 days to respond. In most cases, you will want to request a hearing. Applicable regulations require that your Request for Hearing be filed in a specific form and include certain information (as set out under 21 C.F.R. § 1316.47(a). A Show Cause hearing is conducted by a DEA Administrative Law Judge. These procedures are conducted in accordance with the Administrative Procedures Act (21 C.F.R. §§ 1316.41-1316.68).
Depending on the facts, you may choose to waive your right to a hearing. Even if that is the case, you will still need to file a written position statement within the 30-day response deadline. Under this scenario, if you have submitted a position statement to be considered, it will be forwarded along with the evidence assembled by the agency to the Administrator. The DEA Administrator will then issue a Final Order based on the information and evidence forwarded by the parties.
Finally, you may decide not to contest the action and not to submit a request for a hearing or a position statement. In such a case, the ALJ assigned to your case will base his decision solely on the evidence assembled by the agency. He will then likely issue a Final Order affirming the agency’s decision to deny your application for DEA Registration.
VII. What Happens in a DEA Show Cause Hearing?
The purpose of an Order to Show Cause hearing is for an ALJ to review factual evidence regarding the issues involved in your registration denial6. Parties to the hearing present their case or defense by oral and documentary evidence, submit rebuttal evidence, and cross-examine adverse witnesses. Arguments are offered into evidence and presented in opening and closing statements of counsel. The ALJ will also review proposed findings of fact and conclusions of law submitted by the parties. The burden of proof is on the DEA to show, by a preponderance of the evidence, that a violation occurred and that your application for Registration should be denied.
VIII. Suspension of a Practitioner’s DEA Registration.
Pursuant to 21 U.S.C. § 824(d)(1), the Attorney General (through the DEA Administrator), can suspend a registrant’s DEA Certificate of Registration in cases where there is an “imminent danger to the public health and safety.”[7] If a registrant’s DEA Certificate of Registration is suspended, it will continue in effect until administrative and / or judicial proceedings have been completed. During this period, a suspended registrant will not have the authority to prescribe controlled substances. Moreover, a registrant who is suspended on this basis is not eligible to submit a proposed Corrective Action Plan.
IX. Revocation of a Practitioner’s DEA Registration.
A recent amendment to the law (called the Ensuring Patient Access and Effective Drug Enforcement Act of 2016) now allows recipients of an Order to Show Cause (except those also receiving an Immediate Suspension Order) to submit a Corrective Action Plan. A Corrective Action Plan provides an opportunity for the registrants to demonstrate remedial actions contemplated or taken, and requests DEA to discontinue the Order to Show Cause proceedings. What should be discussed or admitted in a Corrective Action Plan, as well when it should be submitted (the law is unclear), is a decision that likely should be made after seeking advice from an experienced attorney.
X. What is a DEA Registration Corrective Action Plan?
As a result of the passage of the Ensuring Patient Access and “Effective Drug Enforcement Act of 2016,”[8] DEA registrants issued an Order to Show Cause became eligible to submit a Corrective Action Plan.[9] A Corrective Action Plan is intended to show the DEA that a registrant intends to take remedial steps that will obviate the need for Order Show Cause proceedings. Pursuant to 21 U.S.C. § 824(c)(3):
Upon reviewing any corrective action plan submitted by an applicant or registrant pursuant to paragraph (2), the Attorney General shall determine whether denial, revocation, or suspension proceedings should be discontinued, or deferred for the purposes of modification, amendment, or clarification to such plan.
Unfortunately, the statute does not set out how a Corrective Action Plan is to be constructed or discuss how the DEA is to evaluate the plan.